Immunomonitoring Core
Immunomonitoring examines the immune response in the body and is a technique for monitoring patients with inflammation. At Houston Methodist, the Immunomonitoring Core will help researchers and clinicians study the interaction between the immune system and cancer cells, as well as in other research areas like neuroscience or respiratory diseases where multiple markers may be viewed at the same time. In some instances, immunomonitoring is required to assess the effectiveness of a medical intervention or to identify or predict adverse reactions. Immunomonitoring may be considered to be any technical approach or assay that gives information on the immune response of an individual. Future use of these core services may also include RNA and single cell sequencing.
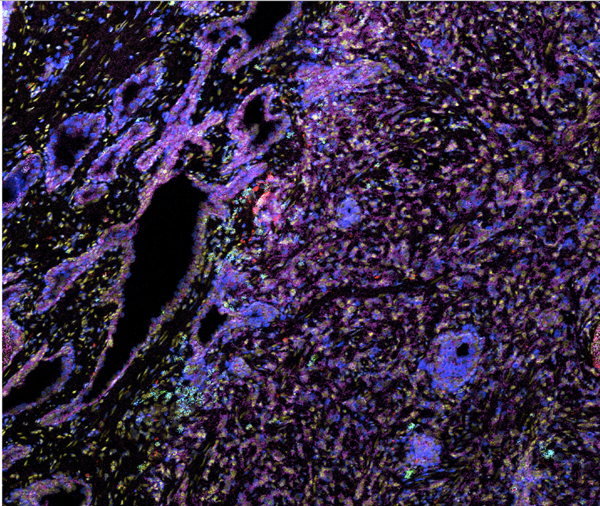
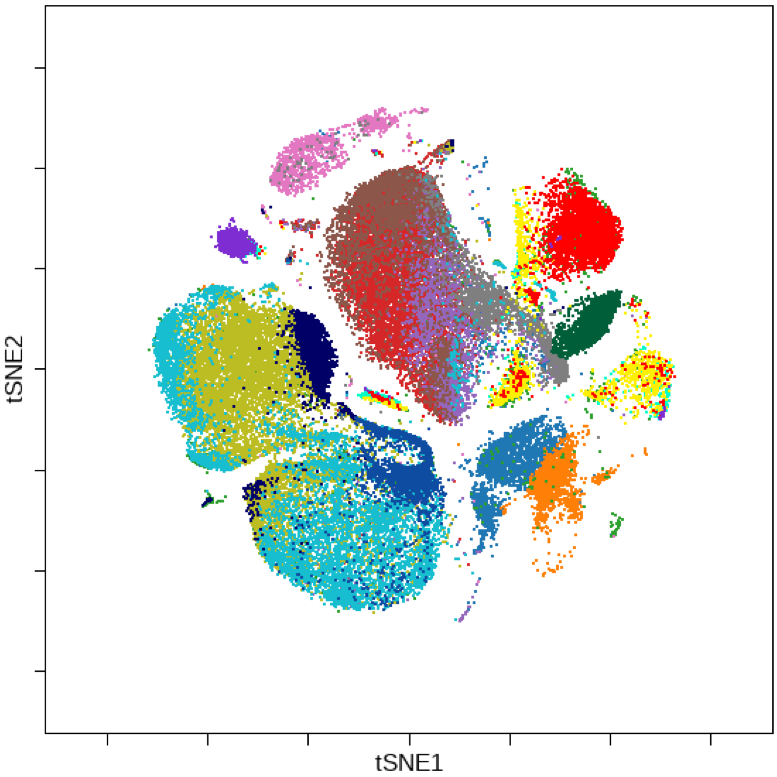
Biomarker discovery efforts in translational medicine are increasingly reliant on antibody-mediated approaches for protein detection in both suspension and fixed tissue sections. Using the Helios™ CyTOF system and Hyperion™ Imaging System, the Houston Methodist Immunomonitoring Core will facilitate immune phenotyping – a technique to study the protein expressed by cells – and spatial analysis of the tumor microenvironment. The imaging system enables simultaneous interrogation of 4 to 37 protein markers using proven CyTOF® technology together with imaging capability.
The use of highly pure metal labels on antibodies in place of fluorescent tags provides a solution to the current challenges in multiplexed tissue imaging by separating signals based on differences in mass instead of wavelength to overcome the limitations of fluorescence-based detection modalities.
The Hyperion Imaging System makes it possible to deeply interrogate tissues and tumors at subcellular resolution while preserving the information in tissue architecture and cellular morphology, ideal for characterization of the tissue microenvironment across a breadth of disease research areas. The Helios systems focuses on suspension samples at cellular level to reveal the whole profile of cellular components within a tumor/disease environment.
Biomarker discovery efforts in translational medicine are increasingly reliant on antibody-mediated approaches for protein detection in both suspension and fixed tissue sections. Using the Helios™ CyTOF system and Hyperion™ Imaging System, the Houston Methodist Immunomonitoring Core will facilitate immune phenotyping – a technique to study the protein expressed by cells – and spatial analysis of the tumor microenvironment. The imaging system enables simultaneous interrogation of 4 to 37 protein markers using proven CyTOF® technology together with imaging capability.
The use of highly pure metal labels on antibodies in place of fluorescent tags provides a solution to the current challenges in multiplexed tissue imaging by separating signals based on differences in mass instead of wavelength to overcome the limitations of fluorescence-based detection modalities.
The Hyperion Imaging System makes it possible to deeply interrogate tissues and tumors at subcellular resolution while preserving the information in tissue architecture and cellular morphology, ideal for characterization of the tissue microenvironment across a breadth of disease research areas. The Helios systems focuses on suspension samples at cellular level to reveal the whole profile of cellular components within a tumor/disease environment.
Services that we provide
Bioinformatics
Biology
- Mass Cytometry (CyTOF)
- Imaging Mass Cytometry (IMC)
- RNAseq
- Single Cell RNAseq (scRNAseq)
- Spatial Transcriptomics (ST)
Biology
- Mass Cytometry (CyTOF)
- Imaging Mass Cytometry (IMC)
- RNAseq
- Single Cell RNAseq (scRNAseq)
- Spatial Transcriptomics (ST)
Meet The Team


Jie Yang
Immune Assessment Core Technician
jyang@houstonmethodist.org

Shu-Hsia Chen, PhD
Emily Herrmann Chair in Immunology Research, Cancer Center
Director, Center for Immunotherapy Research
schen3@houstonmethodist.org
Emily Herrmann Chair in Immunology Research, Cancer Center
Director, Center for Immunotherapy Research
schen3@houstonmethodist.org

