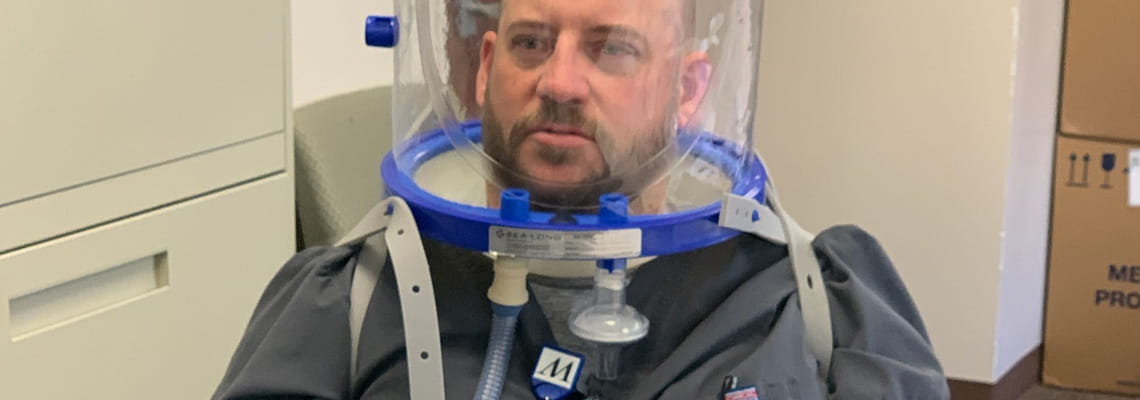
Innovative Helmet May Help COVID-19 Patients Avoid Intubation
View the helmet user guide here >
Tuesday, April 14, 2020 - Only the most severe COVID-19 patients require a stay in the ICU, when they display respiratory symptoms, often from pneumonia. While their care begins with nasal cannula oxygen, it may quickly progress to intubation, which is when a tube is inserted through the nose or mouth to help patients breathe—hence requiring a ventilator. This process has several drawbacks. In addition to putting health care providers at risk when they perform the intubation procedure, some individuals require reintubation after the breathing tube is removed.
With these concerns in mind, Houston Methodist's Faisal Masud, MD, medical director, Center of Critical Care, and professor of clinical anesthesiology, Departments of Anesthesiology & Critical Care and Cardiovascular Sciences, DeBakey Heart & Vascular Center, began seeking clinical alternatives to ventilators for COVID-19 patients. One innovative device was brought to his attention by Houston Methodist Chief Academic Officer, Dirk Sostman, MD, who first learned about a potential device on March 31 and immediately acquired three devices.
Called simply “the helmet,” the device is made of lightweight, transparent plastic that looks like a spacesuit hood. It seals tightly at the neck with two tubes, which supply oxygen while removing carbon dioxide. Produced by Sea-Long Medical Systems in Waxahachie, Texas, the helmets access a hospital's regular oxygen supply. Because the helmet produces a seal around the face, the patient can receive significantly increased oxygenation levels, thus reducing the potential future need for a ventilator. In response to COVID-19, these devices now include a viral filter to minimize secondary exposure for members of the health care team.
After receiving these three helmets, Houston Methodist’s respiratory therapy team began testing the devices to confirm they met specific requirements: a secure seal, an easily attached viral filter and the ability to provide 60 liters of oxygenation. In addition to being more comfortable for patients, the helmet also allows easy access for giving inhaled medicines.
Led by Dr. Masud, his staff will use these helmets for ICU patients who display symptoms from low oxygenation levels due to COVID-19. His team hopes this device will stabilize the patient’s clinical condition and allow for more rapid improvement. Ideally, it also may allow the patient to avoid intubation.
The helmet also will be used in another group of COVID-19 patients. When someone has been extubated, the helmet will be used for 48 hours. This clinical strategy may prevent the need for re-intubation—a common concern for COVID-19 patients during the critical, first 48 hours after they are taken off ventilators.
Sea-Long's helmet, the only one in the U.S. that meets Food and Drug Administration requirements, has been validated in a clinical study for acute respiratory syndrome. The Journal of the American Medical Association published one of the original studies about the device, led by Bhakti Patel, MD, a pulmonologist at the University of Chicago, in 2016. Testing the Sea-Long helmet on ICU patients with acute respiratory distress, she found they needed ventilation only 18.2% of the time, compared to 61.5% of individuals who received their oxygen via face masks. A similar device has also been used by physicians in Italy in their treatment of COVID-19 patients.
As more information becomes available, experts are beginning to question the efficacy of ventilators. For example, recent reports indicate that 80% of New York City patients on ventilators did not recover. This suggests that the use of ventilators may be contributing additional stress for patients with compromised lungs.
Citation: Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. Patel BK1, Wolfe KS1, Pohlman AS1, Hall JB1, Kress JP1. JAMA. 2016 Jun 14;315(22):2435-41. DOI: 10.1001/jama.2016.6338.
