An Interconnected Campus and Culture of Collaboration Accelerates Concepts to the Clinic
Oct. 7, 2020It’s all in the service of bringing innovative medical treatments more efficiently to patients
Houston Methodist is home to thousands of scientists, researchers, engineers, compliance officers, and regulatory experts, where teams are encouraged to collaborate across specialties, to tackle the most challenging diseases and conditions. The campus also includes the essential services, onsite laboratories, and technology that support the full cycle of development of new treatments and devices. This is all just steps from the hospital where our physicians are treating patients with a spectrum of disorders.
Making rapid strides in restoration of motor function after a stroke
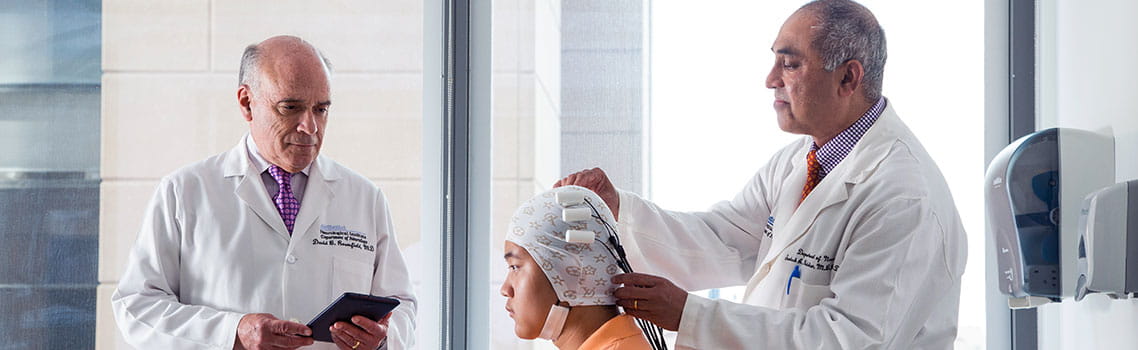
Stroke is the leading cause of disability worldwide. Houston Methodist researchers Santosh Helekar, MD, PhD, and David Chiu, MD, FAHA, of the Helekar & Rosenfield Lab, are collaborating on a revolutionary, noninvasive treatment for stroke patients using a wireless magnetic brain stimulation cap called the Transcranial Rotating Permanent Magnet Stimulator (TRPMS) (invented by Dr. Helekar and Henning Voss, PhD of Weill Cornell Medical College). With the device, HMH’s Helekar and Chiu are showing promise in restoring motor function by using changing magnetic fields to simultaneously stimulate multiple, selected parts of the brain. The portable, affordable device runs on a small rechargeable battery and can be controlled by a smartphone app.
Using nerve stimulation and rehabilitation to contain the damage from a stroke
Inspired by recent studies suggesting that vagus nerve stimulation and rehabilitation could potentially offer a protective effect in treating severe stoke, Philip J. Horner, PhD, and Gavin W. Britz, MBBCh, MPH, MBA, FAANS, collaborated to solidify the evidence. Their work has shown that vagus nerve stimulation decreases stroke infarct volume in preclinical studies, and that targeted electrical stimulation can help activate the brain’s defensive response against stroke. Their work has resulted in a novel treatment, a neural stimulation induced molecular protective therapy, or nSIM, to help contain stroke-related damage. In addition, to avoid interfering with standard-of-care stroke procedures, their nSIM device is incorporated within an endotracheal tube.
Interdisciplinary teams collaborate to meet trial needs
The Phase IIa trial for a drug to treat chronic stable heart failure required 28-hour intervention and observation of each patient. When an enrolled patient became ill 1 week before the trial, it spurred a coordinated effort between departments and critical care teams to bring the trial to success, while providing outstanding patient care.
Houston Methodist’s DeBakey Heart & Vascular Center clinical research team—with the post-anesthesia care unit (PACU), the coronary intensive care unit (CCU), and the investigational drug service—worked beyond their regular hours and responsibilities to fulfill the trial requirements. This included providing all the interventions, sitting bedside through the night, assisting with data collection, and providing resources throughout the trial operations.
Inventing a test to help predict a common cause of liver transplant failure
R. Mark Ghobrial, MD, PhD, Director of the Sherrie and Alan Conover Center for Liver Disease and Transplantation collaborated with Xian Chang Li, MD, PhD, Director of the Immunobiology and Transplant Science Center to create a test that can predict the probability of developing sepsis, a common cause of liver transplant failure. The goal is to help physicians avoid transplants that will ultimately be unsuccessful. The test has shown 95% accuracy in preclinical studies and is currently in early phase clinical trials. If successful, the test has the potential impact to save over $20 billion per year.
Instead of leaving patients vulnerable, regulatory T cells may protect against ALS progression
An interdisciplinary team of researchers and physicians are investigating the etiology and pathophysiology of ALS, led by Stanley H. Appel, MD, Co-Director of the Houston Methodist
Neurological Institute, Peggy & Gary Edwards Distinguished Endowed Chair, and director of the institute and chair of the Stanley H. Appel Department of Neurology. The Edwards ALS Research Laboratory is also part of the effort.
Originally, researchers David Beers, PhD, Associate Research Professor of Neurology and Jenny Henkel, PhD, hypothesized that the common culprit in ALS, and potential target for intervention, is neuroinflammation caused by innate immune microglia and T cells within the adaptive immune system. They developed an ALS mouse model that lacks T cells, hypothesizing that they would exhibit less neuroinflammation and be protected from developing ALS. Instead, ALS progression worsened.
Preclinical studies conducted by Beers and Henkel revealed a specific subpopulation of T-cells called regulatory T cells (Tregs) which are known to stifle inflammation, were shown to suppress ALS progression when expanded outside the body and reinfused, dramatically slowing disease and prolonging survival.
At Houston Methodist, a multifaceted hub of clinicians, analysts, regulatory experts, compliance officers, and a full spectrum of support services are vital to the efficient development of medical innovations. Our streamlined, collaborative culture is the engine that drives new ideas and helps bring them more swiftly through development, resulting in progress for patients.