Oncomagnetic Device a Promising Non-Invasive Weapon Against Deadly Brain Cancer Cells
March 21, 2023 - Eden McCleskeyWith survival statistics ranging from poor to abysmal, primary malignant brain tumors are notoriously aggressive and difficult to treat.
But scientists at Houston Methodist believe they may have finally discovered an Achilles heel that allows them to target and destroy deadly brain cancer cells using oscillating magnetic fields.
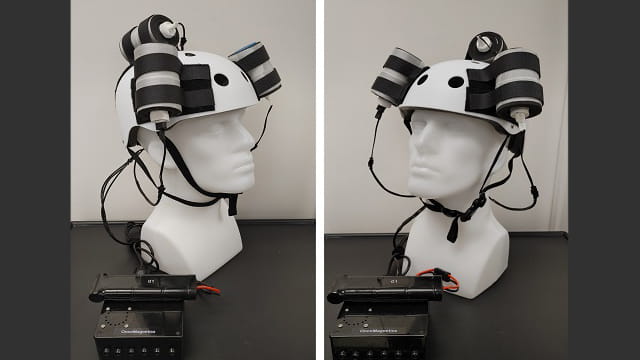
The researchers have created a novel device, dubbed an "oncomagnetic helmet," that has shown remarkable promise at shrinking treatment-resistant brain tumors noninvasively and without systemic side effects.
It works by using powerful magnets to generate targeted frequencies and patterns that disrupt the unique metabolic processes within the mitochondria of cancer cells, triggering apoptosis and the progressive death of nearby cancer cells while leaving healthy cells and tissue unaffected. See the process in action.
It may sound too good to be true, but patient Stephany Gonzalez is living proof that the helmet can work.
Diagnosed at age 28 with a diffuse intrinsic pontine glioma (DIPG), a fatal form of primary brain cancer that typically affects children and young adults, Gonzalez was told she had approximately three months to live.
Today, six months after enrolling in the experimental Houston Methodist trial, there's almost no trace of her DIPG, and her survival window has been extended to "indefinite."
Significant potential
"We've never seen anything like this, and quite honestly, we didn't expect it to work as well as it appears to work," says Dr. David Baskin, director of the Kenneth R. Peak Brain and Pituitary Tumor Treatment Center at Houston Methodist. "It gives us hope that we may be onto something big — a whole new way to fight cancer that may lead to treatment without chemotherapy, radiation, surgery and the collateral damage they can cause."
Dr. Baskin isn't the only one who sees big potential. The Congress of Neurological Surgeons presented him with the Southeastern Brain Tumor Foundation Award in 2022 for the research, and the American Association of Neurological Surgeons (AANS) named him the winner of the prestigious James Rutka Pediatric Brain Tumor Award in 2023.
Also, as the team's initial case study and related safety and background studies continue to generate buzz amongst peers, Dr. Baskin has been asked to present multiple lectures on the topic at the AANS Annual Scientific Meeting in April.
"Interest in this treatment has really skyrocketed lately, I think because we have more data now, we have a patient who is not just surviving but thriving, and there's really strong science backing it all up," Dr. Baskin explains. "When you see these tumors that went from fatally invasive to imploded mush in a matter of weeks, it's hard to argue with that."
How it works
Engineered by Dr. Santosh Helekar, director of the Magnetic Stimulation Device Core Laboratory at the Houston Methodist Research Institute, and his colleagues, the oncomagnetic helmet is a comfortable, portable device that patients can take home and wear while reading or watching TV.
Unlike other experimental magnetic devices, the helmet works without direct contact, so there's no scalp irritation and patients don't have to shave their head. Under current Houston Methodist protocol, patients wear it for two hours at a time, twice or three times a day — a significant quality-of-life advantage over other devices intended to be worn at least 20 hours a day.
The oncomagnetic helmet takes advantage of cancer's distinctive metabolic profile to target only malignant cells.
"Cancer has a different metabolism from other cells," Dr. Baskin says. "It doesn't use glucose or go through the Krebs cycle, it goes through the energy factories in the cell called the mitochondria. That gives us an opportunity to disrupt processes critical to cancer cells' survival without impacting normal healthy cells."
Through trial and error, the scientists identified a specific pattern of oscillating magnetic frequencies focused and powerful enough to alter electron flow in the electron transport chain. This suppresses energy production in the cancer cell, disrupts homeostasis and destabilizes the integrity of the mitochondrial network. It also causes the release of signaling molecules cytochrome C and Smac/DIABLO, which trigger a molecular cascade that leads to lysis and the demise of other cancer cells in the area.
The initial inspiration for this approach came from an unlikely source — General Norman D. Schwarzkopf.
"I had the opportunity to go hunting with him a long time ago, and I asked him what his secret to success was," says Dr. Baskin. "He said if you cut off the enemy's energy supply, you've won the war. No power means no water, no electricity, no communication, and no progress. I've always kept that in the back of my mind. Glioblastoma is a formidable enemy, but that simple concept has driven a lot of my research."
Early results
Although glioblastoma has been Dr. Baskin's primary research focus for over 30 years, his interest in oscillating magnetic fields as a form of treatment is a relatively recent development.
In 2019, he and a small team of core investigators including Martyn Sharpe, Ph.D., Kumar Pichumani, Ph.D., Shashank Hambarde, Ph.D., and resident magnetics expert Dr. Helekar launched the oncomagnetic device initiative for glioblastoma patients. By 2020, they had completed the current helmet prototype, conducted in vitro and animal model preclinical trials and begun a compassionate-use clinical trial.
The first patient to undergo the therapy, a 53-year-old male with recurrent end-stage glioblastoma, passed away mid-treatment from an unrelated complication of the disease, but his autopsy showed the promise: His tumor volume had reduced more than 30% after only six weeks of treatment.
Over the next two years, five more end-stage glioblastoma patients experienced similar results: tumor volumes were reduced by 30%-60% despite the disease state being too advanced for the patients to overcome.
In the fall of 2022, the team petitioned the FDA to approve the helmet's use for Gonzalez, the first non-glioblastoma patient to undergo the treatment.
Her rare adult-onset case of DIPG featured a particularly invasive brain-stem tumor which could not be completely resected and is unresponsive to chemotherapy and radiation.
"It's the same type of glial cell but not the same type of tumor — DIPG has a very specific molecular marker that is even more pernicious than glioblastoma," Dr. Baskin explains. "But it is still energized by mitochondria and has the same mechanism of action, so we were hopeful that the device would work just as well on it."
In fact, this time it worked even better, reducing the size of the tumor by nearly 100% while Gonzalez continued to go to work and live her normal life.
"Although her tumor was equally life-threatening, Stephany was in much better condition when she entered treatment than the end-stage glioblastoma patients who had already endured months or years of aggressive treatments," says Dr. Baskin. "Her success with this treatment bodes well for other DIPG patients and less advanced glioblastoma cases once we are able to expand beyond expanded access/compassionate use exemptions, into a full-scale clinical trial."
Next steps
Dr. Baskin and team are currently in the process of acquiring the necessary funds to launch the next phase of their research, and they are hoping to initiate both a local and international clinical trial within the next year.
Meanwhile, they have an even more pressing clinical concern — figuring out what to do with Gonzalez now that she has thoroughly defied expectations.
"Should we continue the same course or reduce the dose; when will it be safe to discontinue treatment," Dr. Baskin ponders. "We're in uncharted territory now, writing the rules as we go. But it's an enviable problem to have, and I wouldn't want it any other way."